Stem cell markers as a resource to predict prognosis of betel quid induced oral squamous cell carcinoma: an immunohistochemical investigation
Abstract
Aim: The study was to correlate the survival of a group of Sri Lankan betel quid induced oral cancer patients with the expression patterns of five cancer stem cell (CSC) markers namely ALDH1, Sox-2, Bmi-1, C-met, and Oct-4.
Methods: Archival tissues of 140 oral squamous cell carcinomas (OSCCs) were stained with CSC markers, ALDH1, Sox-2, Bmi-1, C-met, and Oct-4. Survival analysis was performed with SPSS version 20, using Kaplan-Meier survival curves and significance with chi-square test and Breslow’s (generalized Wilcoxon) test.
Results: Immunohistochemical investigations revealed 55.7%, 58.6%, 40%, 34.6%, and 31.4% of OSCCs showing ALDH1, Sox-2, Bmi-1, C-met and Oct-4 expression respectively. ALDH1 positivity was significantly associated with nodal metastasis (X2 = 4.6; P = 0.03). Although it did not reach statistical significance, ALDH1 expression was also correlated with poor survival (P = 0.06). No statistically significant findings could be observed when the expression of other CSC markers were correlated with nodal metastasis and survival (P > 0.05).
Conclusion: Out of the five CSC markers investigated, only ALDH1 showed promise as a potential marker to predict nodal metastasis and survival in the present study sample of OSCC patients.
Keywords
Introduction
Oral squamous cell carcinoma (OSCC) is the commonest malignancy, in Sri Lanka, contributing to 19.5% of all cancers detected in both sexes.[1] Betel chewing and use of other forms of tobacco are the main causes for the high incidence of OSCC in South Asia. The risk of oral cancer is increased with the combined effect of betel chewing and smoking rather than betel chewing or smoking alone. The habit of betel chewing and smoking accounts for 75% of cancers in larynx, pharynx and oral cavity in Sri Lanka.[1] Older males in lower socio-economic groups and ethnic minority groups are frequently affected from OSCC.[1] Low-income and disadvantaged groups are usually affected from cancers due to exposure to avoidable risk factors such as environmental carcinogens, tobacco use, alcohol and infectious agents. These groups also do not have sufficient knowledge to protect and improve their health.
In Sri Lankan, patients who practice the habit of betel quid chewing, OSCC more commonly occurs on the buccal mucosa, while the tongue is the second commonest site of occurrence for OSCC.[1] However, compared to OSCC that occurs in the buccal mucosa, OSCC of the tongue shows a higher propensity to develop nodal metastasis.[1] Unlike other malignancies that affect different body sites, survival rates of patients with OSCC have not improved significantly over the years. Therefore, desire to improve the survival by developing methods to enhance the diagnostic and treatment efficacy of OSCC as well as identification of novel markers to predict metastasis and recurrences exist.
Cancer stem cell (CSC) is defined as a subpopulation of cells that exhibit self-renewal capacity with the ability to produce heterogeneous lineage of cells that comprise of the tumor.[2] Thus, CSC in addition to demonstrating self-sustainability, possess the ability to differentiate and regenerate. Further, CSC also demonstrates resistance to radiotherapy and chemotherapy.[3,4]
To date, CSC has been isolated in hematopoietic malignancies, solid tumors of the breast, brain, prostate, lung, colon, liver, skin, head and neck and melanoma.[2] The CSC markers that have been identified in OSCC include, CD44,[2] ALDH,[2,5] CD133,[2,6] Sox-2,[2,7] Oct-4,[2,8] C-met,[2,8] Bmi-1.[9] In OSCC, CSC identified by different markers has been correlated with radioresistence, high tumorigenic activity, high tumor initiation activity and with clinicopathological features.[10,11] Thus, immunohistochemistry based expression patterns of different CSC markers would be useful to predict the biological behavior of the OSCC and the final outcome.
The aim of the study was to correlate the survival of Sri Lankan OSCC patients with the expression patterns of five CSC markers namely ALDH1, Sox-2, Bmi-1, C-met, and Oct-4.
Methods
A previously tested questionnaire was used to obtain information regarding the habit history, the present status of the tumor and survival details of patients with OSCC diagnosed during a period of 4 years from 2009 to June 2012. One hundred and forty OSCC patients (relatives when the patient had died), who responded to the questionnaire were selected for the study.
The 140 patients with a positive habit history of betel chewing were included in the study.
Regarding the habit of betel chewing all patients had chewed betel for more than 10 years (range 11-61 years). The number of quids chewed ranged from 2-8 per day. In addition, the betel quid of all patients had included both known carcinogens tobacco and areca nut. Out of the 103 males, 69 (67%) males smoked either cigarettes or beedi, while 35 (34%) had used alcohol for more than 10 years on a daily basis. Twenty-nine (28%) males had practiced all 3 habits on a daily basis for more than 17 years.
Archival OSCC tissues from these patients were used for the immunohistochemical investigations with CSC markers, ALDH1, Sox-2, Bmi-1, C-met and Oct-4 performed according to the manufacturer’s instructions (Abcam Pvt Ltd, UK). The primary antibodies were applied in 1:200, 1:500, 1:250, 1:5000, and 1:250 dilutions for ALDH1, Sox-2, Bmi-1, C-met and Oct-4 respectively. Normal lung tissue, human skin, hepatocellular carcinoma, tonsil and bronchial carcinomas were used as positive controls for ALDH1, Sox-2, Bmi-1, C-met and Oct-4 CSC markers respectively. Ten high power fields with the most number of positive cells were identified and counted with the average taken as the final score. A tumor was considered to express the CSC maker when > 5% of the OSCC cells gave a positive reaction while tumors are expressing < 5% of positive cells were considered as negative. Survival analysis was performed with SPSS version 20, using Kaplan-Meier survival curves and significance with chi-square test and Breslow’s (generalized Wilcoxon) test.
Results
According to Table 1, the study sample comprised of 9.3% patients younger than 45 years while the rest were older than 46 years at the time of diagnosis. Male to female ratio was 2.8:1. Buccal mucosa was the commonest site of occurrence, contributing to 67.8% of OSCC, followed by tongue (23.6%). The majority (80%) of OSCC patients had tumour-node-metastasis (TNM) stage III or IV disease at diagnosis with 28.6% of patients presenting with pathological confirmed nodal metastasis. With reference to survival data gathered using the questionnaire, survival of patients included in the study sample ranged from 2-120 months. Twenty-eight patients had succumbed to the disease, after surviving for 2-60 months. Out of these patients, majority (22 patients) had died within 12 months of the surgery.
The clinicopathological characteristics of the study sample and the cancer stem cell marker expression patterns in the oral squamous cell carcinoma
| Feature | Total number of cases (%) | ALDH+ | Sox-2+ | Bmi-1+ | C-met+ | Oct-4+ |
|---|---|---|---|---|---|---|
| Age | ||||||
| < 45 years | 13 (9.3) | 4 | 7 | 5 | 0 | 4 |
| 46-60 years | 68 (48.6) | 36 | 40 | 27 | 15 | 15 |
| > 61 years | 59 (42.1) | 38 | 35 | 24 | 16 | 25 |
| χ2 | P = 0.07 | P = 0.93 | P = 0.09 | P = 0.10 | P = 0.90 | |
| Gender | ||||||
| Female | 37 (26.4) | 18 | 22 | 14 | 9 | 15 |
| Male | 103 (73.6) | 60 | 60 | 42 | 27 | 34 |
| χ2 | P = 0.30 | P = 0.89 | P = 0.41 | P = 0.80 | P = 0.7 | |
| Site | ||||||
| Buccal mucosa | 95 (67.8) | 55 | 60 | 49 | 28 | 30 |
| Tongue | 33 (23.6) | 20 | 16 | 15 | 6 | 10 |
| Other | 12 (8.6) | 3 | 6 | 2 | 2 | 4 |
| χ2 | P = 1.0 | P = 0.2 | P = 0.9 | P = 0.20 | P = 0.54 | |
| Stage | ||||||
| Stage I/II | 28 (20.0) | 11 | 12 | 10 | 5 | 5 |
| Stage III/IV | 112 (80.0) | 67 | 70 | 46 | 31 | 39 |
| χ2 | P = 0.08 | P = 0.20 | P = 0.90 | P = 0.20 | P = 0.70 | |
| Metastasis | ||||||
| Present | 40 (28.6) | 28 | 22 | 21 | 12 | 17 |
| Absent | 100 (71.4) | 50 | 60 | 35 | 24 | 27 |
| χ2 | P = 0.03* | P = 0.50 | P = 0.07 | P = 0.40 | P = 0.32 |
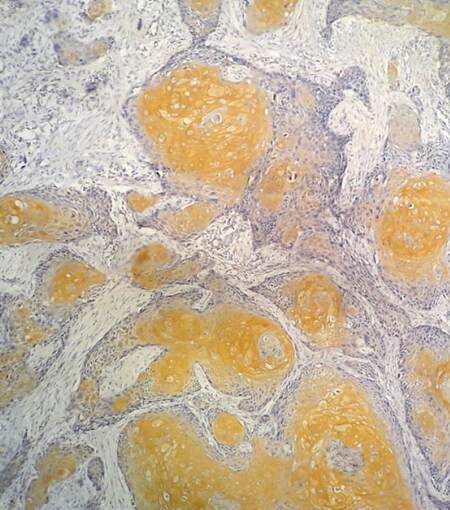
Immunohistochemical investigations revealed 55.7%, 58.6%, 40%, 34.6%, and 31.4% of OSCCs showing ALDH1 [Figure 1], Sox-2, Bmi-1, C-met and Oct-4 expression respectively. Cytoplasmic positivity of ALDH1, Sox-2, Bmi-1; membrane and cytoplasmic positivity of C-met and nuclear positivity of Oct-4 were considered as true positivity. ALDH1 expression was significantly correlated with nodal metastasis (X2 = 4.6; P = 0.03).
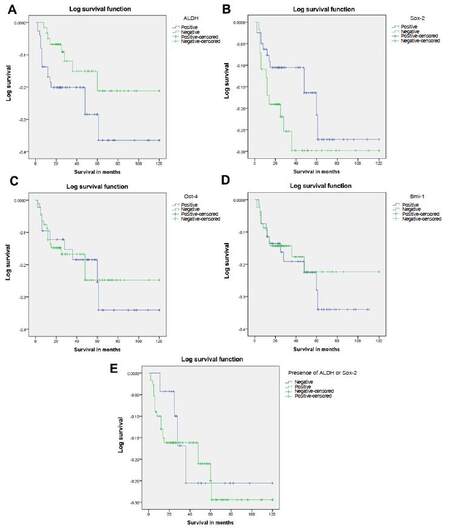
Although it did not reach statistical significance, ALDH1 expression was also correlated with poor survival (P = 0.06) [Figure 2A]. No statistically significant findings could be observed when the expressions of other CSC markers were correlated with nodal metastasis and survival (P > 0.05) [Table 1][Figure 2B-D].
Figure 2. (A) Survival differences in patients who had OSCC which expressed ALDH (indicated in blue) compared to ALDH negative tumours (indicated in green). Note: relatively poor survival of patients who had cancers which expressed the marker; (B) survival differences in patients who had OSCC which expressed Sox-2 (indicated in blue) compared to Sox-2 negative tumours (indicated in green). Note: there are no significant survival differences in tumours with and without Sox-2 expression; (C) survival differences in patients who had OSCC which expressed Oct-4 (indicated in blue) compared to Oct-4 negative tumours (indicated in green). Note: there are no significant survival differences in patients with and without Oct-4 expression; (D) survival differences in patients who had OSCC which expressed Bmi-1 (indicated in blue) compared to Bmi-1 negative tumours (indicated in green). Note: no significant difference in survival is observed in patients with and without Bmi-1 expression; (E) survival differences in patients who had OSCC which expressed ALDH and Sox-2 (indicated in green) compared to ALDH and Sox-2 negative tumours (indicated in blue). Note: there are no significant survival differences between patients who had tumours with and without cancer stem cell marker expression. OSCC: oral squamous cell carcinoma
Discussion
CSC were first diagnosed in leukemic patients in 1994.[12] From the time of identification, CSC has been subjected to intensive studies. Currently, major focus is on properties of CSC and mechanisms of their formation. Identification of CSC has been performed by using different types of CSC markers.[13]
Cancer stem cell hypothesis formulated to explain the tumorigenic process is composed of the following key features: (1) only a small fraction of cancer cells within a tumor has tumorigenic potential; (2) cancer stem cell populations can be differentiated from other cancer cells by distinctive surface markers; (3) tumors resulting from cancer stem cells have both tumorigenic and non-tumorigenic cells of the original tumor; (4) cancer stem cell populations can be serially transplanted through multiple generations indicating self-renewal capacity.[14] Recent evidence also suggests that CSC is generally resistant to conventional therapy and are the initiators of recurrences and metastatic spread.
Therefore, the present research was undertaken to establish the presence of CSC in a group of OSCC patients from Sri Lanka, where majority of the cancers occur due to the habit of betel quid chewing. All patients included in the study had practiced betel chewing for more than 10 years. In addition, majority of males (67%) were smokers, while 34% used alcohol on a daily basis. Results revealed that all 5 CSC markers evaluated were expressed in approximately 35-55% of OSCC depending on the marker. As such results of the present study confirm the fact that all 5 markers can be used to evaluate stem cell potential in OSCC.[5,7-9]
Michifuri et al.[5] has found a correlation between high ALDH1 positive rate in tumor cells and lymph node metastasis. According to their study, more than 2% of ALDH1 expression rate was correlated with nodal metastasis. In this study, more than 5% of ALDH1 expression level correlated with nodal metastasis. Other clinical and pathological factors analyzed did not show positive correlation with ALDH1 expression. Therefore, the study also confirms the fact that ALDH1 expression could be used to predict nodal metastasis in OSCC. Although, other studies show that combined ALDH1 and Sox-2 expression as predictor of poor survival, our results do not show similar findings [Figure 2E].[5,7] However, when only ALDH1 expression is considered, although it did not reach statistical significance, majority of the tumors which expressed this marker occurred in patients who showed poor survival. Lack of statistical significance may have occurred due to low sample size.
In contrast to the results of the present study, previous studies have shown poor survival in OSCC expressing Sox-2, C-met, Bmi-1, and Oct-4.[15-18] Etiological differences may have contributed to this result. In addition, majority of the tumors in the present study sample contained buccal mucosal cancers; while the literature quoted above have focused on tongue cancers.[16,18] Furthermore, Sox-2 expression has been shown to be related to poor survival at sites such as esophagus and bladder as well.[19,20] In addition, problems highlighted below concerning survival data may have also contributed to this result.
Problems encountered when gathering survival information of patients can be considered as one of the major limitation of this study. Although, we used a previously tested questionnaire to gather this information, in spite of sending reminders, most of the responses that we received were from patients who had survived with treatment. Therefore, the study sample showed survival bias and cannot be considered as an ideal sample. In addition, the percentage of patients who responded to the questionnaire was approximately 50%. Therefore, we had to restrict our sample to 140 patients. Furthermore, when we tried to correlate CSC expression with survival in patients with and without radiotherapy, we found that the information gathered from the questionnaire to be inadequate. As such, we were able to identify the modifications required for the questionnaire in the future.
Immunohistochemistry is an important application for identification of the specific antigens that are distributed in the tissue sections. The monoclonal antibodies, as well as polyclonal antibodies, are specific to the particular antigens are used with this technique.[21] In this study, the paraffin embedded tissue sections were immunohistochemically stained to identify the expression of CSC in OSCC. After the correct fixation and processing, the formalin fixed paraffin embedded tissue blocks should be stored properly. The tissues that have been embedded in paraffin are stable for extended periods. Many laboratories have routinely performed immunohistochemical investigations on 20-year-old material without generating any problems.[21] The archival samples of OSCC that were received by the Department of Oral Pathology since 2009 to 2012 were used for this study. The sections that were taken from the specimens processed in 2012 were better stained compared to older samples. However, it did not affect the interpretation of results.
In conclusion, out of the five CSC markers investigated in the present study, only ALDH1 showed promise as a potential marker to predict nodal metastasis and survival in the present study sample of OSCC patients.
Authors’ contributions
Manuscript’s preparation: P. Jayasooriya
Manuscript’s review: U. Dissanayake
Concept design: P. Jayasooriya
Literature search: C. Fernando
Immunohistochemical investigations: A. Suraweera
Acknowledgments
We thank Dr. Jon Wagner for his contribution in language editing and Dr. Dayan Anuruddha for his contribution in statistical assistance.
Financial support and sponsorship
University Peradeniya Research grant 2013/16-D.
Conflicts of interest
There are no conflicts of interests.
Patient consent
Not applicable.
Ethics approval
Ethical clearance for the study was obtained from the Faculty of Dental Science, Ethics and Review Committee.
REFERENCES
1. Cancer incidence data: Sri Lanka Year 2001-2005. Available from: http://www.nccp.health.gov.lk/images/PDF_PUBLICATIONS/Cancer_Incidence_Data_2005.pdf. [Last accessed on 15 Mar 2017].
2. Major AG, Pitty LP, Farah CS. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int 2013;2013:319489.
3. Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistence. Nat Rev Cancer 2008;8:545-54.
4. Damek-Poprawa M, Volgina A, Korostoff J, Sollecito TP, Brose MS, O'Malley Jr BW, Akintoye SO. Targeted inhibition of CD133+ cells in oral cancer cell lines. J Dent Res 2011;90:638-45.
5. Michifuri Y, Hirohashi Y, Torigoe T, Miyazaki A, Kobayashi J, Sasaki T, Fujino J, Asanuma H, Tamura Y, Nakamori K, Hasegawa T, Hiratsuka H, Sato N. High expression of ALDH1 and SOX-2 diffuse staining pattern of oral squamous cell carcinoma correlates with lymph node metastasis. Pathol Int 2012;62:684-89.
6. Zhang Q, Shi S, Yen Y, Brown J, Ta JQ, Le AD. A subpopulation of CD133+ cancer stem like cells characterized in oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett 2010;289:151-60.
7. Du L, Yang Y, Xiao X, Wang C, Zhang X, Wang L, Zhang X, Li W, Zheng G, Wang S, Dong Z. Sox-2 nuclear expression is closely associated with poor prognosis in patients with histologically node negative oral tongue squamous cell carcinoma. Oral Oncol 2011;47:709-13.
8. Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlates of Oct-4 and Nanog in oral cancer stem cells and high grade oral squamous cell carcinoma. Clin Cancer Res 2008;14:4085-95.
9. Yu CC, Lo WL, Chen YW, Huang PI, Hsu HS, Tseng LM, Hung SC, Kao SY, Chang CJ, Chiou SH. Bmi-1 regulates snail expression and promotes metastasis ability in head and neck squamous cancer derived ALDH1 positive cells. J Oncol 2011;2011.
10. Mitra D, Malkoski SP, Wang XJ. Cancer stem cells in head and neck cancer. Cancers (Basel) 2011;3:415-27.
11. Rajapakshe RM, Pallegama RW, Jayasooriya PR, Siriwardena BS, Dias DK, Attygalle AM, Hewapathirana S, Weerasinghe JU, Tilakaratne WM. A retrospective analysis to determine factors influencing the management of patients with oral squamous cell carcinoma. Cancer Epidemiol 2015;39:360-6.
13. Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug 2014;13:497-512.
15. Yoshihama R, Yamaguchi K, Imajyo I, Mine M, Hiyake N Akimoto N, Kobayashi Y, Chigita S, Kumamaru W, Kiyoshima T, Mori Y, Sugiura T. Expression levels of SOX2, KLF4 and brachyury transcription factors are associated with metastasis and poor prognosis in oral squamous cell carcinoma. Oncol Lett 2016;11:1435-46.
16. Habu N, Imanishi Y, Kameyama K, Shimoda M, Tokumaru Y, Sakamoto K, Fujii R, Shigetomi S, Otsuka K, Sato Y, Watanabe Y, Ozawa H, Tomita T, Fujii M, Ogawa K. Expression of Oct3/4 and Nanog in the head and neck squamous carcinoma cells and its clinical implications for delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma. BMC Cancer 2015;15:730.
17. He Q, Liu Z, Zhao T, Zhao L, Zhou X, Wang A. Bmi1 drives stem-like properties and is associated with migration, invasion, and poor prognosis in tongue squamous cell carcinoma. Int J Biol Sci 2015;11:1-10.
18. Huang CF, Xu XR, Wu TF, Sun ZJ, Zhang WF. Correlation of ALDH1, CD44, OCT4 and SOX2 in tongue squamous cell carcinoma and their association with disease progression and prognosis. J Oral Pathol Med 2014;43:492-8.
19. Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, Ramos AH, Woo MS, Weir BA, Getz G, Beroukhim R, O'Kelly M, Dutt A, Rozenblatt-Rosen O, Dziunycz P, Komisarof J, Chirieac LR, Lafargue CJ, Scheble V, Wilbertz T, Ma C, Rao S, Nakagawa H, Stairs DB, Lin L, Giordano TJ, Wagner P, Minna JD, Gazdar AF, Zhu CQ, Brose MS, Cecconello I, Ribeiro U Jr, Marie SK, Dahl O, Shivdasani RA, Tsao MS, Rubin MA, Wong KK, Regev A, Hahn WC, Beer DG, Rustgi AK, Meyerson M. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 2009;41:1238-42.
20. Kitamura H, Torigoe T, Hirohashi Y, Asanuma H, Inoue R, Nishida S, Tanaka T, Fukuta F, Masumori N, Sato N, Tsukamoto T. Prognostic impact of the expression of ALDH1 and SOX2 in urothelial cancer of the upper urinary tract. Mod Pathol 2013;26:117-24.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Jayasooriya P, Fernando C, Suraweera A, Dissanayake U. Stem cell markers as a resource to predict prognosis of betel quid induced oral squamous cell carcinoma: an immunohistochemical investigation. Stomatological Dis Sci 2017;1:29-34. http://dx.doi.org/10.20517/2573-0002.2016.11
AMA Style
Jayasooriya P, Fernando C, Suraweera A, Dissanayake U. Stem cell markers as a resource to predict prognosis of betel quid induced oral squamous cell carcinoma: an immunohistochemical investigation. Stomatological Disease and Science. 2017; 1: 29-34. http://dx.doi.org/10.20517/2573-0002.2016.11
Chicago/Turabian Style
Jayasooriya, Primali, Champika Fernando, Anura Suraweera, Upul Dissanayake. 2017. "Stem cell markers as a resource to predict prognosis of betel quid induced oral squamous cell carcinoma: an immunohistochemical investigation" Stomatological Disease and Science. 1: 29-34. http://dx.doi.org/10.20517/2573-0002.2016.11
ACS Style
Jayasooriya, P.; Fernando C.; Suraweera A.; Dissanayake U. Stem cell markers as a resource to predict prognosis of betel quid induced oral squamous cell carcinoma: an immunohistochemical investigation. Stomatological. Dis. Sci. 2017, 1, 29-34. http://dx.doi.org/10.20517/2573-0002.2016.11
About This Article
Copyright
Author Biographies

Data & Comments
Data
 Cite This Article 3 clicks
Cite This Article 3 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.